The ABC of Hydrogen
September 29, 2020
PowerUP is part of the hydrogen revolution happening right now
November 8, 2020The ABC of Fuel Cells
Approximately 46% of the electricity generated worldwide comes from the combustion of fossil fuels. However, these resources won’t last forever as the increasing population is eating up the fossil fuels reserve faster than ever. One solution to tackle this situation is fuel cell technology.
Although considered the future of energy generation, fuel cells have been around for decades. Even centuries. In 1800, William Nicholson and Anthony Carlisle described the process of using electricity to break water into hydrogen and oxygen. The invention of fuel cells is credited to Sir William Grove in 1839, whose “Grove cell” generated about 12 amps of current at approximately 1.8 volts.
In 1932, Francis Thomas Bacon successfully developed a 5 kW stationary fuel cell. The alkaline fuel cell (AFC), also known as the Bacon fuel cell, is one of the most developed fuel cell technologies, which NASA has used since the mid-1960s.
So, what exactly is a fuel cell?
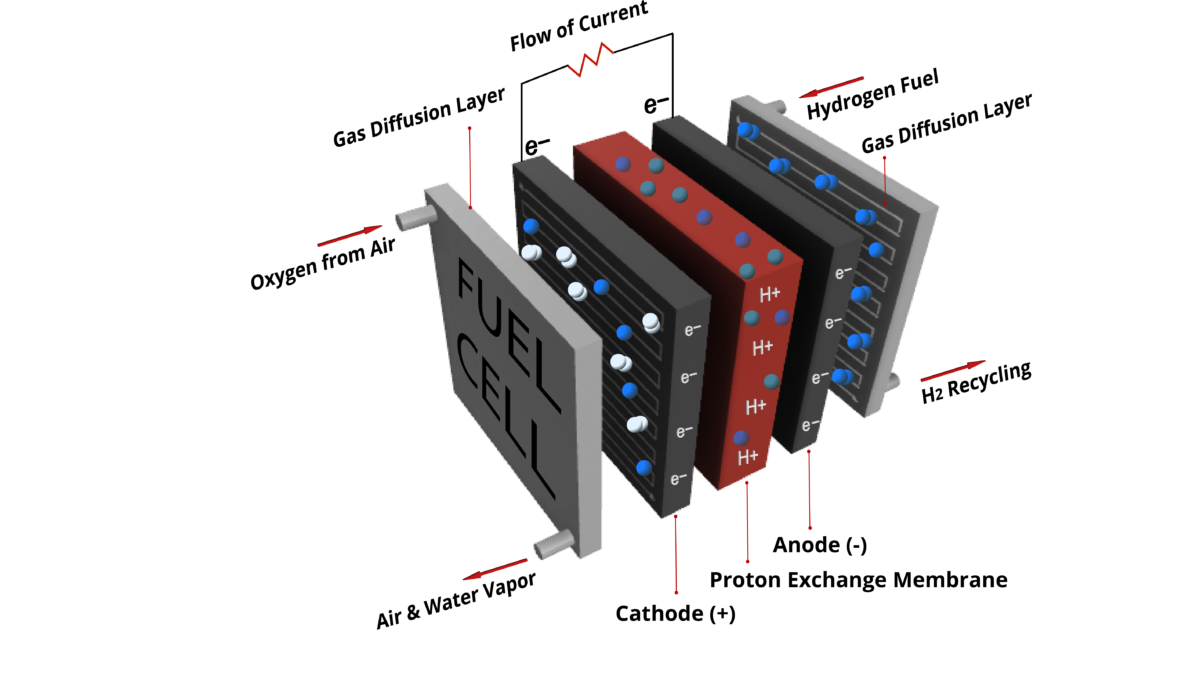
A fuel cell is a device that generates electricity by a chemical reaction, which requires hydrogen and oxygen. Every fuel cell has two electrodes called – anode and cathode. The reactions that produce electricity take place in the electrodes. Fuel cells also have an electrolyte, which carries electrically charged particles from one electrode to the other, and a catalyst to speed the reactions at the electrodes. A single fuel cell generates a tiny amount of direct current (DC) electricity. In practice, many fuel cells are usually assembled into a stack.
Types of fuel cells
While there are different types of fuel cells, they all generally work in the same manner. Below are the most common types of fuels cells used widely across
- Proton exchange membrane (PEM) fuel cells – They operate at a low temperature, which makes them suitable for homes, cars and other similar applications. Typically, platinum catalyst is used, which drives the cost.
- Solid oxide fuel cells (SOFC) – A solid, ceramic compound material is used as an electrolyte. They require high operating temperatures (800-1000 °C). SOFC’s can run on a variety of fuels including natural gas. Due to high temperatures, their applications are limited.
- Alkaline fuel cells (AFC) – Their efficiency is about 70% with an operating temperature from 150 to 200 °C. Alkaline fuel cells were used in Apollo missions to provide electricity and drinking water.
- Phosphoric Acid fuel cells (PAFC) – As the name suggests, they use phosphoric acid as the electrolyte. Different types of fuels can be used and their efficiency ranges from 40 to 80%.
- Molten carbonate fuel cells (MCFC) – They require a high operating temperature (about 650 °C). They hold several advantages over other fuel cell technologies, including their resistance to impurities.
Applications
Now that we know the different types of fuel cells, let’s look at their applications. According to different estimates, the fuel cell market is growing at a CAGR of 15-34% during 2020-2026. Applications include:
- power generation,
- cogeneration (CHP – combined heat and power),
- fuel cell electric vehicles (FCEVs) – there are roughly 15,000 fuel cell cars on the roads as of October 2020,
- buses, trains, forklifts, airplanes, motorcycles, boats, submarines and other modes of transportation,
- portable or emergency power systems.
Stationary fuel cells are the largest, most powerful fuel cells. They are designed to provide a clean, reliable source of on-site power to hospitals, banks, airports, military bases, schools, and homes.
Advantages
Unlike a typical battery, which eventually goes dead, a fuel cell continues to produce energy as long as fuel and oxygen are supplied. Today, hydrogen fuel cells promise two to three times the efficiency of traditional combustion technologies. Fuel cells provide:
- a 50% reduction in fuel consumption compared to a conventional vehicle with gasoline internal combustion engine;
- zero to near-zero levels of harmful emissions from vehicles and power plants;
- quiet operation;
- fewer moving parts;
- scalability, and the use for a variety of applications including transportation, stability and portable power.
Fuel cells are appealing because they offer high efficiency, excellent part-load performance, lower emissions of regulated pollutants, and a wide size range.
Drawbacks
Today, the biggest hurdle is cost. This is mainly due to the high cost of platinum catalysts. Albeit hydrogen is the most abundant element in the universe, it proves difficult to store and distribute. The infrastructure does not support the mass adoption of fuel cell technologies just yet.
The future of fuel cells looks very bright with new initiatives and collaborations between many nations based on the mutual need for energy and the replacement of fossil fuels. While rapid technical advancements are being made, fuel cells will be an integral part of our everyday lives sooner rather than later.